Abstract
Background: A lack of scalable experimental platforms that adequately recapitulate human bone marrow (BM) has hampered the study of normal and malignant hematopoiesis, as well as the validation of novel therapies. Although organoid technologies are rapidly advancing research in other fields, organoids that models the complexity of native BM have not yet been reported. Our aim was to engineer human induced pluripotent stem cell (iPSC)-derived organoids with homology to human BM, and to assess their utility for modelling blood and BM disorders.
Methods: A directed differentiation protocol giving rise to mesenchymal stromal cells (MSCs), endothelial cells (ECs) and myeloid cell subtypes was developed and characterized by single cell RNA-sequencing, multi-modal imaging and functional studies. Architectural and transcriptional homology to human BM was assessed, and the utility of the system as a model for normal and perturbed hematopoiesis was determined, including the ability to support engraftment and survival of primary cells from healthy donors and patients with blood cancers.
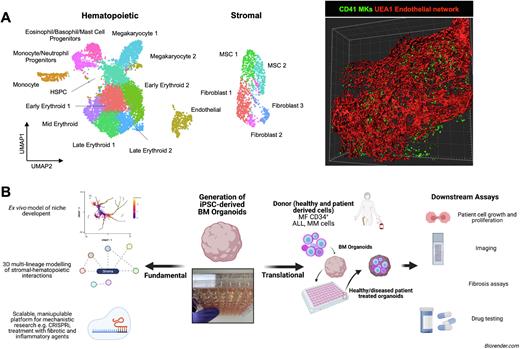
Results: Hydrogel composition and growth factor supplementation were optimised to support the generation of self-organizing, vascularised 3D cultures with cellular, molecular and architectural homology to the central myelopoietic perivascular niche. Organoids were composed of MSCs, fibroblasts, sinusoidal ECs, as well as myelomonocytic, erythroid, megakaryocytic, and eo-baso-mast cells (Fig. 1A). 3D imaging revealed a dense network of lumen-forming vessels invested with MSCs/fibroblasts (Fig. 1A), with perivascular megakaryocytes (MKs) and myeloid cells migrating to the sinusoidal lumens. Comparison to primary human tissue revealed immunophenotypic and transcriptional specialization of ECs and MSCs to resemble BM sinusoidal endothelium and MSCs respectively. Endogenous production of stem cell factor, GM-CSF, FLT3L, CD40L and other hematopoiesis-promoting cytokines and chemokines by organoids was confirmed at the protein level. Strong autocrine and paracrine receptor-ligand interactions were detected between hematopoietic cells and their stroma, recapitulating regulatory interactions occurring in the BM niche, including CXCL12, NOTCH1, FGF, KITL and ANGPT2, PDGF and TGFβ and interleukin signalling pathways.
Having demonstrated homology to human BM and confirmed the capacity of our system to generate supporting growth factors, we hypothesized that the organoids could provide a platform for the growth and maintenance of primary adult BM cells, and mimic cancer-induced niche remodelling. Seeding of organoids with CD34+ cells from healthy donors and patients with myelofibrosis resulted in the rapid engraftment, proliferation and differentiation of donor cells within the organoids, as well as the maintenance of a CD34+, quiescent stem/progenitor population. Myelofibrosis CD34+ cells, but not cells from healthy donors, induced fibrotic remodelling of the organoid niche (collagen deposition and myofibroblast activation). Fibrosis was inhibited by treatment with JQ1, a potent BET bromodomain inhibitor, but not ruxolitinib, a JAK1+2 inhibitor widely used in myelofibrosis treatment that, while reducing splenomegaly and symptoms in a majority of patients, does not typically improve fibrosis. Dose-dependent induction of hallmarks of fibrosis in organoids also occurred following TGFβ treatment without patient cells, enabling the robust screening of candidate inhibitors of fibrosis.
We next assessed whether the organoids could support survival and proliferation of hematologic cancer cells that are particularly challenging to maintain ex vivo. We found significantly improved viability and proliferation of cells from patients with myeloid and lymphoid malignancies including multiple myeloma and acute lymphoblastic leukemia over 12 days of culture as compared to both liquid culture and the previous gold standard, 3D co-culture with human BM MSCs, and the cells maintained their immunophenotype.
Conclusion: We describe a novel technology for the study of normal and perturbed hematology that we hope will be transformative in mechanistic studies of hematopoiesis and in modelling BM disorders (Fig. 1B), and accelerate target discovery, validation and translation.
Disclosures
Fielding:Amgen: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Gooding:Bristol Meyers Squibb: Research Funding. Machlus:STRM.BIO: Consultancy, Current holder of stock options in a privately-held company. Psaila:Evotec: Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; Blueprint Therapeutics: Consultancy; Constellation Therapeutics: Consultancy; Galecto: Research Funding; Alethiomics: Consultancy, Current equity holder in private company, Honoraria, Other: Co-founder , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal